What Is the Electron-pair Geometry for N in No2cl
The electron-pair geometry around the N atom in the NO3- molecule is. So according to it NF3 central atom contains 1 lone pair.

No2cl Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
The electron-pair geometry around the N atom in NOCl.
. 1 lone pair around the central atom x 1. The shape of a PCl3 molecule is Trigonal pyramidal. 1045 because the lone pairs are larger and more diffuse than a localized bonding pair and therefore more likely to influence structure.
The geometry of valence. There isare lone pairs around the central atom so the molecular geometry of the NOCl molecule is predicted to be. The central P atom has one lone pair of electrons and three bond pairs of electrons.
25 For the Lewis Structure. 2 Which of the moleculesions have a triangular planar electron-pair geometry. Lets see how to find the molecular and electron geometry of NF3 this step by step.
Electron pair geometry is tetrahedralMolecular shape ia pyramidal. The electron-pair geometry around the N atom in NICI IS There are lone pairs around the central Natom so the geometry of the NICI molecule is predicted to be Submit Answer Retry Entire. I think the electronic geometry is trigonal planar and its polar.
We can arrive at this answer because. Geometry is determined by the number of electron groups. The electron pair geometry would be trigonal planar because there is a lone pair on the oxygen atom.
What are the electron-pair geometry and molecular geometry around the central atom. A 2 NOBrg. Do not include overall ion charges or formal charges in your drawing.
Is for space O-N-O Cl I distributed 6 dots around the 2 Os and Cl but N has one valence electron on top and it is unevenly distributed. The electron-pair geometry is determined by the positions of the bonds and lone pairs in a molecule. H O H is compressed from the ideal tetrahedral angle of 1095 to approx.
It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and Trigonal pyramidal molecular geometry. Up to 256 cash back What is the electron-pair geometry and the molecular geometry around the central atom. A CH 2 F 2 b ICl 4- c SbF 6- d SnCl 5- e XeO 2 F 2.
With this we can find out that it can. The molecular geometry of the AsO2- ion is bent because of the lone electron pair with the central arsenic atom making the O-As-O bond. The geometry of NOBr is bent and the molecule is polar.
Draw the Lewis structure for carbonyl fluoride COF 2. Around each oxygen atom there are 2 lone pairs and 2 bonding pairs of electrons to form the O H bonds. Here A central atom X surrounding atoms and E lone pair of electrons around the central atom.
____ What is the the. With NO 2 Cl youll need to form a double bond between one of the Oxygen atoms and a Nitrogen atom to fill the octets and still use only the 24 valence electrons available for the molecule. The electron-pair geometry for N in NOBr is trigonal planar.
In VSEPR theory the shape or geometry of a molecule is determined by electron-electron repulsion. 1 Which of the moleculesions have an octahedral electron-pair geometry. A N has 6 valence electrons O has 62 12 valence electrons Cl has 7 valence electrons Total.
Nitrogen N is the least electronegative atom and goes at the center of the NO 2 Cl Lewis structure. Nitronium ion NO2 is a nonpolar molecule because the molecule deposit two N-O bonds are polar. The geometry of the electron pair around the Boron atom in is called the trigonal planarIn this case there is a pair of isolated electrons around Boron which is the central atom allowing the molecule to have a folded geometry.
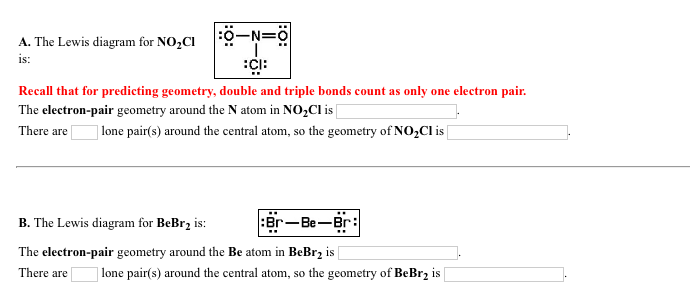
Do not draw double bonds to oxygen atoms unless they are needed for the central atom to obey. What is the electron-pair geometry for N in NO2Cl. 3 Which of the moleculesions have a triangular bipyramidal electron-pair.
Question 12 4 Points Write the equilibrium constant expression K for the following reactions. The molecular pair geometry would be bent. The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures â It is made up of one Phosphorus atom and five Fluorine atoms.
In NOCl A is Nitrogen we have one O and one Cl around Nitrogen n 2 and there are two lone electrons ie. What is the electron-pair geometry around the N atom in NO2Cl. Number of electron groups Name of electron group geometry 2 linear 3 trigonal-planar 4 tetrahedral 5 trigonal-bipyramidal 6 octahedral Molecular geometry on the other hand depends on not only on the number of electron groups but also on the number of lone pairs.
Draw the Lewis structure for NO2Cl in the window below and then answer the questions that follow. When the electron. Such as when SODIUM combines with CHLORINE to form NaCl then one electron out of 7 valence electrons of chlorine get combined with one valence electron of sodium but still there are 6 valence electrons in the outermost shell of Cl and these are called unshaired electrons and their pair is known as lone pair electronsAnd there are 3 lonepair electrons in Cl in this case of NaCl.
Find the Number of lone pairs present on the central atom of the NF3 lewis structure. The electron-pair geometry around the Natom in the NO2Cl molecule is lone pairs around the central Natom so the geometry of the NO2Cl molecule is predicted to be There are B. The required VSEPR notation for nitrosyl chloride is AX2E1.
What is the electron-pair geometry around the C atom in CH4. To find the number of lone pairs you can use the Nitrogen trifluoride lewis structure. Boron is an element that has three electrons in its valence layer.
A BCl 3 b NO 2- c NOCl d SbF 3 e SO 3.

No2cl Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

No2cl Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Solved The Lewis Diagram For No 2cl Is Recall That For Chegg Com
No comments for "What Is the Electron-pair Geometry for N in No2cl"
Post a Comment